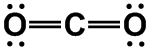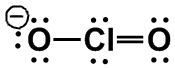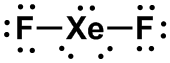VSEPR and Molecular Shapes |
|
 |
Valence Shell Electron Pair Repulsion (VSEPR) Theory is a simple, qualitative model that allows the prediction of an approximate molecular shape, given a valid Lewis structure of a molecule. As such, VSEPR is an extremely powerful tool, because molecular shape offers insight into a wide range of important physical properties (polarity, solubility, volatility, chirality, etc). VSEPR theory treats each pair of electrons at an atom – either bonds or lone pairs – as a localized region of electron density, directed outward from the atomic centre. The predicted molecular shape will be such that the bonds and lone pairs are arranged so as to minimize repulsions and maximize the space among them.
|
| Advantages |
Disadvantages |
• based on a simple, qualitative premise
• fast and intuitive
• qualitatively accurate
• especially useful for organic compounds
|
• requires a valid Lewis structure, so limited to molecules for which Lewis structures are useful
– no transistion-metal complexes
– no odd-electron molecules
– many structures not correctly predicted by simple Lewis model
• offers only approximate, qualitative predictions for non-symmetric molecules
• the simple premise is a gross oversimplification of the underlying physical principles
|
The basic arrangements of the electron pairs depends on the number of regions of electron density (bonds + lone pairs), as follows:
Regions of
Electron Density |
2 |
3 |
4 |
5 |
6 |
| Arrangement |
linear
|
trigonal planar
|
tetrahedral
|
trigonal bipyramidal
|
octahedral
|
| Bond Angles |
180° |
120° |
109.5° |
120° and 90° |
90° |
| |
|
|
|
|
|
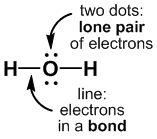
The shape of the molecule can differ from the basic arrangement of electron density regions, depending on how many "corners" of the arrangement are taken up by lone pairs. A common notation is used to classify various molecules and the shapes they should adopt: AXnEm, where A = central atom, X = atoms connected to A by bonds, E = lone pairs on A.
The resulting molecular geometry depends only upon the positions of the atoms, and will differ from the electron pair arrangement if there are lone pairs on the central atom.
How to Determine a VSEPR Structure
1) Draw the Lewis Structure.
2) Count the bonds and lone pairs about the central atom. Note that multiple bonds are counted as one region of electron density.
3) Determine the basic arrangement of all electron density regions (bonds + lone pairs) about the central atom.
4) Determine the molecular geometry based on resulting positions of atoms.
| Basic Arrangement |
Lone Pairs |
Notation |
Molecular Shape |
Examples |
linear
(2 bonds or lone pairs) |
0 |
AX2 |
LINEAR
|
CO2, BeF2, [N3]–
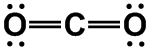 |
trigonal
planar
(3 bonds or lone pairs) |
0 |
AX3 |
TRIGONAL PLANAR
|
BF3, [CO3]2–,
[NO3]–
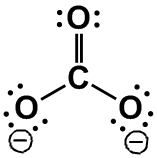 |
| 1 |
AX2E |
BENT
|
SO2, [NO2]–
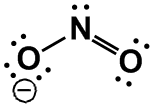
|
tetrahedral
(4 bonds or lone pairs) |
0 |
AX4 |
TETRAHEDRAL
|
CH4, [AlCl4]–,
[NH4]+, [SO4]2–
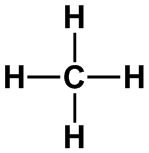
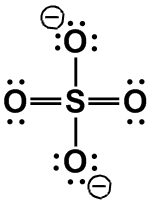 |
| 1 |
AX3E |
PYRAMIDAL
|
NH3, [SO3]2–
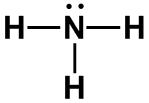
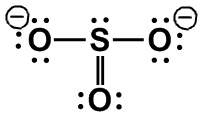 |
| 2 |
AX2E2 |
BENT
|
H2O, [ClO2]–
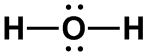
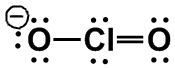 |
trigonal
bipyramidal
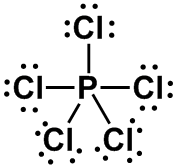
(5 bonds or lone pairs) |
0 |
AX5 |
TRIGONAL BIPYRAMIDAL
|
PCl5
 |
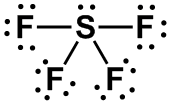
| 1 |
AX4E |
SEE-SAW
|
SF4
 |
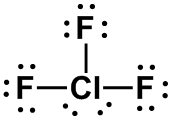
| 2 |
AX3E2 |
T-SHAPED
|
ClF3
 |
| 3 |
AX2E3 |
LINEAR
|
XeF2, [I3]–
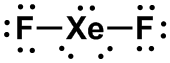 |
octahedral
(6 bonds or lone pairs) |
0 |
AX6 |
OCTAHEDRAL
|
SF6, [PCl6]–
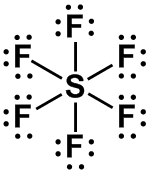 |
| 1 |
AX5E |
SQUARE PYRAMIDAL
|
IF5, BrF5
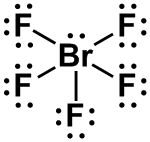 |
| 2 |
AX4E2 |
SQUARE PLANAR
|
XeF4
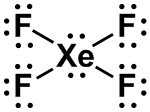 |
Another VSEPR page, including a number of practice problems,
can be found at Purdue
University.
This page is maintained and copyright by W. Stephen McNeil at UBC Okanagan.
All educational works available on this page are licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 2.5 Canada License.