- used for Fe transport in vertebrates (some arthropods, molluscs)
- different types found in different fluids: serum Tf (blood), ovoTf (egg whites), lactoferrin (milk, saliva)
- ~80kDa, one chain, two lobes, each lobe has two domains
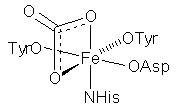
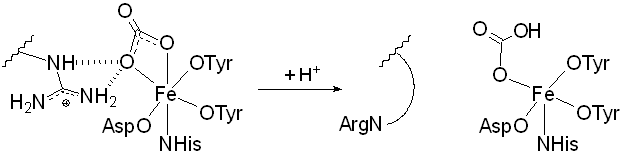
- each lobe binds one Fe(III), octahedral 2 x OTyr NHis OAsp + exogenous bidentate [CO3]2–