biological assembly
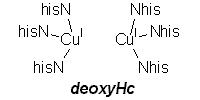
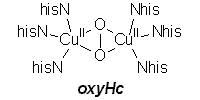
Oxygen Transport Proteins
Oxygen Transport
|
deoxyMyoglobin |
oxyMyoglobin |
deoxyHemoglobin |
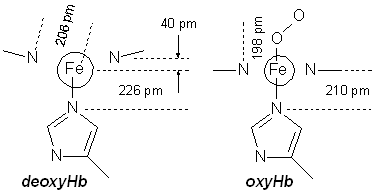
In deoxyMb and deoxyHb, the large HS Fe(II) sits slightly out the plane of the porphyrin ring. Oxygen coordinates in the vacant axial position, and forms a hydrogen bond to another histidine group that points into the binding pocket. Coordination reduces the radius of the iron (forming either LS Fe(II)(O2) or LS Fe(III)(O2•–) depending on who you ask and whether you believe there's a meaningful difference). This reduces the ionic radius of the Fe, allowing it to move into the ring, pulling the bound histidine group with it. In Hb, this movement of the histidine by over 50 pm induces a reorganization of the protein chains in the other subunits, giving rise to a cooperativity effect, whereby binding of one or two O2 groups facilitates oxygenation of all four iron centres.
| deoxyHemocyanin |
oxyHemocyanin |
deoxyHemocyanin: biological assembly |
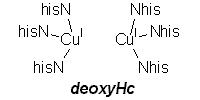 |
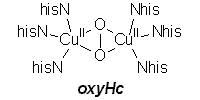 |
| deoxyHemerythrin: active site |
oxyHemerythrin: active site |
deoxyHemerythrin: biological assembly |
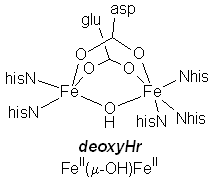 |
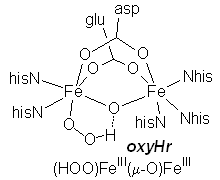 |
This page is maintained and copyright by W. Stephen McNeil at UBC Okanagan.
All educational works available on this page are licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 2.5 Canada License. ![]()