Nitrogenase Proteins
|
Fe protein (electron transfer to MoFe) |
MoFe protein (reduces N2) |
Nitrogenase is an enzyme found in certain bacteria that can transform atmospheric dinitrogen into ammonia. It consists of two separate multi-chain proteins. The Fe protein contains a [4Fe4S] ferredoxin unit that acts as an electron-transfer cofactor, passing electrons from ferredoxin to the MoFe protein. The MoFe protein is the site of N2 reduction, and consists of four subunits, containing two sets of metalloclusters. The clusters are:
|
P Cluster (electron transfer) |
FeMo Cluster (N2 activation) |
The P cluster is bound to the protein by 6 cysteine residues (4 terminal and 2 bridging), so that each Fe atom is tetrahedrally coordinated by sulfur atoms. In effect, this cluster is two [4Fe4S] ferredoxin units that share in common one vertex as a μ6-S atom.
The FeMo cluster is bound to the protein through one Fe by a cysteine sulfur and through the Mo by a histidine. There is also a bidentate homocitrate ligand completing octahedral coordination at Mo. Histidine and arginine sidechains are directed over one face of four iron atoms, and are thought to act as proton shuttle sites to the cluster during catalysis. The cluster consists of two [4M3S] units bridged by three sulfur atoms. High-end spectroscopy and careful reanalysis of crystallographic diffraction data has revealed a μ6-carbon atom in the centre of this cluster. Click the box to view the carbon:
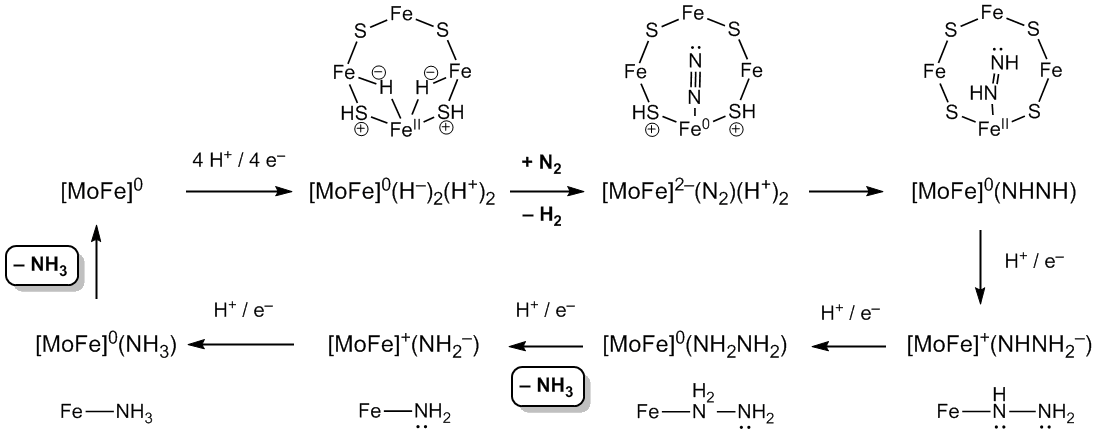
The mechanistic details of the function of this enzyme have taken eighty years to unravel. The Fe protein delivers electrons one at a time into the FeMo cluster, with eight electrons total required to complete one cycle and effect the reduction of one molecule of N2 and two protons to dihydrogen: N2 + 8 H+ + 8 e– ==> 2 NH3 + H2. At a single four-iron face of the FeMo cluster, the first four additions of H+ / e– generate two bridging hydrides and protonate two briding S atoms. Reductive elimination of H2 reduces one iron atom, which then binds and reduces N2 as it is protonated by the nearby [SH]+ groups, yielding Fe-bound azene (HNNH). Subsequent additions of H+ / e– reduce and cleave the N-N bond, via intermediate hydrazine and amido species.

This page is maintained and copyright by W. Stephen McNeil at UBC Okanagan.
All educational works available on this page are licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 2.5 Canada License. ![]()