Chirality, Enantiomers, and R/S Nomenclature
One type of steorisomerism is that of molecules that are chiral. A chiral molecule is non-superimposable on its mirror image, so that the mirror image is a different molecule. The two non-identical mirror images are a pair of enantiomers.* Unlike other sorts of isomers, enantiomers have identical physical and chemical properties (except those properties that involve interaction with other chiral objects). This makes enantiomers difficult to separate, and they are often used or encountered as a 1:1 mixture of the two enantiomers, called a racemic mixture or racemate.
* A common macroscopic example of chirality is your hands. Your hands have the same connectivity of fingers and thumbs, but they are mirror images of each other, and so you cannot superimpose them. The word chiral comes from the Greek χειροϛ, meaning hand. The word enantiomer comes from the ancient Greek έναντιοϛ, meanins opposite.
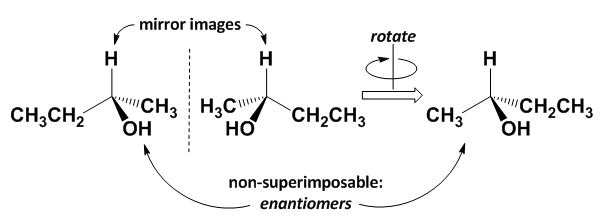
One common origin of chirality (and that most commonly encountered in organic chemistry) is a tetrahedral coordination of four different groups about a central atom, usually a carbon atom. The central atom is referred to as a chiral centre or stereocentre. If any two of the groups bound to a tetrahedral centre are the same, then the molecule has a plane of symmetry, the mirror image is an identical and superimposable molecule, and the two molecules are not enantiomers. This is shown below at left. However, if all four groups are different, the molecule does not have a plane of symmetry, such that the mirror image is non-superimposable, and so it is a different molecule. The result is a pair of two enantiomers, as shown below at right.
|
These molecules are mirror images, but can be superimposed, demonstrating that they are not enantiomers but are in fact the same molecule. Push the button below to see that the two molecules are the same. |
These mirror image molecules are non-superimposable, and so they are enantiomers. Each is a different chiral molecule. | ||
|
|
|
|
|
The two enantiomers of 2-butanol are shown below. The mirror image of the first molecule is positioned such that the -H and -OH groups can be superimposed, but the -CH3 and -CH2CH3 groups are in the reverse positions. No matter how the second moleucle is oriented, no more than two groups can be aligned with the first molecule. These two molecules are non-superimposible mirror images, so they are a pair of enantiomers.

|
Importance of Enantiomers
|
For the most part, enantiomers have identical physical and chemical properties. Nevertheless, the difference between two enantiomers can have enormous impact, particularly in biological systems, because many important biological molecules are chiral. A prominent example is that of amino acids, which are the building blocks of proteins and enzymes, and have the formula +H3NCH(R)CO2–. Note that the central C atom is surrounded by four different groups, so that amino acids are chiral (except in the case of glycine, where R = H). Only one enantiomer of amino acids is used by biological systems to build proteins, so all proteins and enzymes are also chiral structures. |
This enantiomer of the amino acid alanine is found in proteins and enzymes. spin: |
This enantiomer of alanine cannot be used for protein synthesis, but does serve other biological roles. spin: |
|
Thus, the chemistry of your body is controlled by chiral molecules, which means that different enantiomers of a molecule will react with it in different ways. This includes pharmaceuticals: different enantiomers of a chiral drug often exhibit different bioavailabilities, metabolism rate, potency, or toxicity. For example, the (S)-enantiomer of citalopram, used to treat depression, is 30 times as potent as the (R)-enantiomer. A classic example is the drug thalidomide, shown at right, which was sold worldwide to pregnant women as a treatment for morning sickness from 1957-1962, and was responsible for tens of thousands babies with severe birth defects. Thalidomide is chiral: the carbon with four different groups is shown in yellow. There is evidence to suggest that only one of the two isomers is teratogenic in mice, and it is commonly believed that the birth defects could have been avoided if thalidomide had been marketed as an enantiomerically pure substance rather than a racemic mixture. However, even enantiomerically pure thalidomide racemizes in vivo, so that it is effectively impossible to administer just one form. Another classic example of the different physiological behaviour of enantiomers is the odour of limonene. One isomer interacts with your olfactory receptors to provide the scent of oranges, while the other smells of pine and turpentine. |
(R)-thalidomide: suggested non-teratogen spin: (S)-thalidomide: potent teratogen spin: |
|
|
(R)-limonene, scent of orange spin: |
(S)-limonene, scent of pine spin: |
|
R/S Nomeclature of Enantiomers
Enantiomers pose a problem of nomenclature. Two enantiomers have the same atoms connected in the same way, so that following standard IUPAC nomenclature rules will give both compounds the same name. How can we distinguish the two mirror-images? In organic chemistry, the most common origin of chirality is a tetrahedral (sp3) carbon atom bound to four different groups. A system of nomenclature to distinguish the two groups must describes the three-dimensional arrangement of those four groups about the chiral carbon centre:
 |
a | 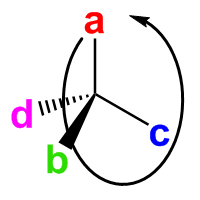 |
|||
| b | |||||
| c | |||||
| d | |||||
|
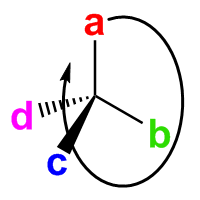
Place the lowest-priority group d behind the carbon. Other groups form a clockwise path from a to b to c. This is the R isomer. |
Place the lowest-priority group d behind the carbon. Other groups form a counter-clockwise path from a to b to c. This is the S isomer. |
||||
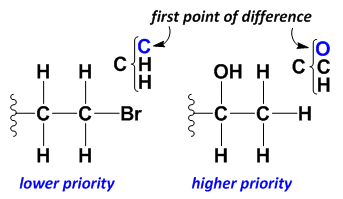
Cahn-Ingold-Prelog Rules for Substituent Ranking
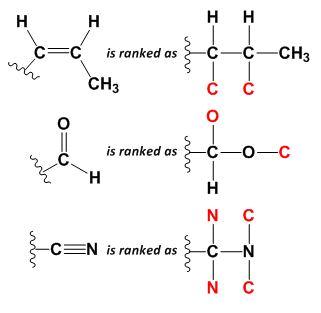
|
This page is maintained and copyright by W. Stephen McNeil at UBC Okanagan.
All educational works available on this page are licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 2.5 Canada License. ![]()